

5, John Wiley & Sons, Chichester, UK, 1996. Harris in Encyclopedia of Nuclear Magnetic Resonance, D.M. Magnetogyric ratio, γ (10 7 rad T ‑1 s -1) Table of NMR-active nucleus propeties of gold
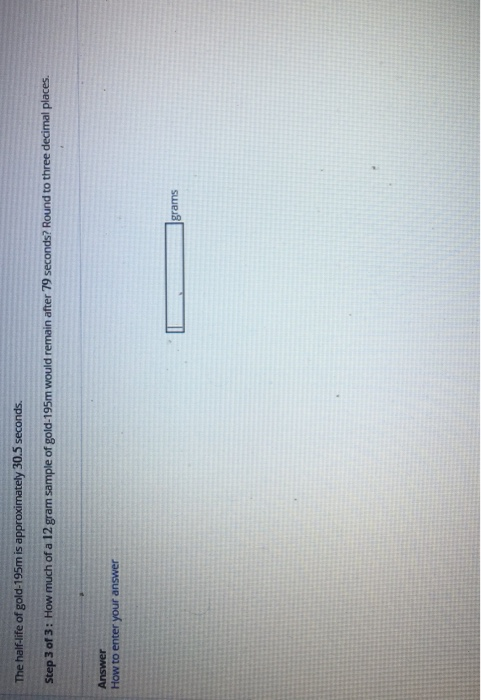

Ĭommon reference compound: no defined reference. Kuchitsu in Quantities, Units and Symbols in Physical Chemistry, Blackwell Scientific Publications, Oxford, UK, 1988. Masses, nuclear spins, and magnetic moments: I.For further information about radioisotopes see Jonghwa Chang's (Korea Atomic Energy Research Institute) Table of the Nuclides.Naturally occurring isotope abundances: Commission on Atomic Weights and Isotopic Abundances report for the International Union of Pure and Applied Chemistry in Isotopic Compositions of the Elements 1989, Pure and Applied Chemistry, 1998, 70, 217.This table gives information about some radiosotopes of gold, their masses, their half-lives, their modes of decay, their nuclear spins, and their nuclear magnetic moments. This relationship enables the determination of all values, as long as at least one is known.Further data for naturally occuring isotopes of gold are listed above. Using the above equations, it is also possible for a relationship to be derived between t 1/2, τ, and λ. Derivation of the Relationship Between Half-Life Constants This means that the fossil is 11,460 years old. If an archaeologist found a fossil sample that contained 25% carbon-14 in comparison to a living sample, the time of the fossil sample's death could be determined by rearranging equation 1, since N t, N 0, and t 1/2 are known. N t is the remaining quantity after time, t The carbon-14 undergoes radioactive decay once the plant or animal dies, and measuring the amount of carbon-14 in a sample conveys information about when the plant or animal died.īelow are shown three equivalent formulas describing exponential decay: It is incorporated into plants through photosynthesis, and then into animals when they consume plants. The process of carbon-14 dating was developed by William Libby, and is based on the fact that carbon-14 is constantly being made in the atmosphere. The half-life of carbon-14 is approximately 5,730 years, and it can be reliably used to measure dates up to around 50,000 years ago. One of the most well-known applications of half-life is carbon-14 dating. The term is most commonly used in relation to atoms undergoing radioactive decay, but can be used to describe other types of decay, whether exponential or not. Half-life is defined as the amount of time it takes a given quantity to decrease to half of its initial value.


 0 kommentar(er)
0 kommentar(er)
